Dmg Chemical Formula
- Dmg Chemical Formula 1
- Dmg Chemical Formula Chart
- Dmg Chemical Formula Reviews
- Dimethylglyoxime
- Dmg Chemical Formula List
| Names | |
|---|---|
| Systematic IUPAC name | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.006.147 |
| EC Number | |
PubChemCID | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| C4H6NiO4 | |
| Molar mass | 176.781 g·mol−1 |
| Appearance | Green Solid |
| Odor | slight acetic acid |
| Density | 1.798 g/cm3 (anhydrous) 1.744 g/cm3 (tetrahydrate) |
| Melting point | decomposes when heated [1][2] |
| Easily soluble in cold water, hot water | |
| Solubility | Soluble in methanol insoluble in diethyl ether, n-octanol |
| +4,690.0·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| P21/c | |
α = 90°, β = 93.6°, γ = 90°[3] | |
Lattice volume (V) | 471.5 |
| 2 | |
| distorted octahedral | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
| 350 mg/kg (rat, oral) 410 mg/kg (mouse, oral)[4] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
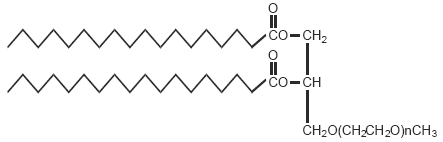
DuPont cares about your privacy. Your personal information (name, email, phone number and other contact data) will be stored in chosen customer systems primarily hosted in the United States. A current list of alchemy formulas. These formulas can be recorded in tomes. The first step of every formula (except extractions) is to LIGHT your cauldron, the last step of every formula (except extractions) is to ALCHEMY SEAL. All of the items that need to be foraged can be found here. An alternate search method can be found here. Molecular Formula. A chemical formula is a way of expressing information about the proportions of atoms that constitute a particular chemical compound, using a single line of chemical element symbols and numbers. PubChem uses the Hill system whereby the number of carbon atoms in a molecule is indicated first, the number of hydrogen atoms second. Dimethylglyoxime is a chemical compound described by the formula CH 3 C(NOH)C(NOH)CH 3. Its abbreviation is dmgH 2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl). DmgH 2 is used in the analysis of palladium or nickel.
Dmg Chemical Formula 1
Nickel(II) acetate is the name for the coordination compounds with the formula Ni(CH3CO2)2·x H2O where x can be 0, 2, and 4. The green tetrahydrate Ni(CH3CO2)2·4 H2O is most common. It is used for electroplating.
DMG supplement sublingual 100 mg, 125 mg 500 mg benefit side effects Dimethylglycine, dosage, tablets, latest information by Ray Sahelian, M.D. December 22 2017. If you find the field of mind-boosting pills, sex nutrients, and anti-aging interesting, you will certainly want to learn more about DMG (dimethylglycine), TMG (trimethylglycine), and methyl donors. The latest research on Dimethylglycine. Though DMG is a highly effective supplement taken alone, there are reports of athletes competing at the Olympic Games in Sydney 2000 combining it with phosphates, which themselves have been shown to boost endurance. /latest-studies-of-dmg.html.
Synthesis and structure[edit]
The compound can be prepared by treating nickel or nickel(II) carbonate with acetic acid:
- NiCO3 + 2 CH3CO2H + 3 H2O → Ni(CH3CO2)2·4 H2O + CO2
Dmg Chemical Formula Chart
The green tetrahydrate has been shown by X-ray crystallography to adopt an octahedral structure, the central nickel centre being coordinated by four water molecules and two acetate ligands.[5] It may be dehydrated in vacuo, by reaction with acetic anhydride,[6] or by heat.[7]
Safety[edit]
Nickel salts are carcinogenic and irritate the skin.
References[edit]
Dmg Chemical Formula Reviews
- ^M. A. Mohamed, S. A. Halawy, M. M. Ebrahim: 'Non-isothermal decomposition of nickel acetate tetrahydrate', in: Journal of Analytical and Applied Pyrolysis, 1993, 27 (2), S. 109–110. doi:10.1016/0165-2370(93)80002-H.
- ^G. A. M. Hussein, A. K. H. Nohman, K. M. A. Attyia: 'Characterization of the decomposition course of nickel acetate tetrahydrate in air', in: Journal of Thermal Analysis and Calorimetry, 1994, 42, S. 1155–1165; doi:10.1007/BF02546925.
- ^Downie, T. C.; Harrison, W.; Raper, E. S.; Hepworth, M. A. (15 March 1971). 'A three-dimensional study of the crystal structure of nickel acetate tetrahydrate'. Acta Crystallographica Section B. 27 (3): 706–712. doi:10.1107/S0567740871002802.
- ^'Nickel metal and other compounds (as Ni)'. Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^Van Niekerk, J. N.; Schoening, F. R. L. (1953). 'The crystal structures of nickel acetate, Ni(CH3COO)2·4H2O, and cobalt acetate, Co(CH3COO)2·4H2O'. Acta Crystallogr.6 (7): 609–612. doi:10.1107/S0365110X5300171X.
- ^Lascelles, Keith; Morgan, Lindsay G.; Nicholls, David; Beyersmann, Detmar (2005). 'Nickel Compounds'. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_235.pub2.
- ^Tappmeyer, W. P.; Davidson, Arthur W. (1963). 'Cobalt and Nickel Acetates in Anhydrous Acetic Acid'. Inorg. Chem.2 (4): 823–825. doi:10.1021/ic50008a039.
Dimethylglyoxime
| AcOH | He | ||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH | B(OAc)3 | AcOAc ROAc | NH4OAc | AcOOH | FAc | Ne | ||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 | Si | P | S | ClAc | Ar | ||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 | Mn(OAc)2 Mn(OAc)3 | Fe(OAc)2 Fe(OAc)3 | Co(OAc)2, Co(OAc)3 | Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr |
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 | Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 | Sb(OAc)3 | Te | IAc | Xe |
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 | TlOAc Tl(OAc)3 | Pb(OAc)2 Pb(OAc)4 | Bi(OAc)3 | Po | At | Rn | |
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |